38 explain how controlled substances are identified on their labels
WHMIS 2015 - Labels : OSH Answers - Canadian Centre for Occupational ... The slash (/) means the supplier is to specify the appropriate type of equipment based on their knowledge of the product and how it is used. So, for example, this statement could appear as: Wear protective gloves and eye protection. Or, Wear protective gloves. Or, Wear protective gloves, protective clothing, eye protection, and face protection. Controlled Substances Act of 1970: Definition & History A controlled substance is a medication (or drug or substance) that is regulated by the government, including its possession, manufacturing, and sale. The Controlled Substances Act of 1970 (CSA) was...
The 5 Types Of Controlled Substances | Chemical Substance Regulation Controlled Substance Schedules. The schedules for controlled substances range from Schedule I to Schedule V. The lower the schedule, the greater the control is. This means that if someone is caught illicitly using, possessing, or selling a schedule 1 substance, the consequences will generally be greater than that of a schedule 5 substance.
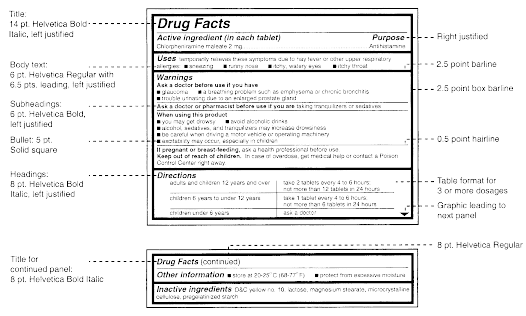
Explain how controlled substances are identified on their labels
Controlled Substances - FAQ - Environmental Health and Safety What are controlled substances? Controlled substances are drugs or chemicals that have the potential to be addictive or habit-forming. The DEA divides controlled substances into 5 categories called Schedules based upon substances' potential for abuse and addictiveness and its' usefulness in medicine. The Drug Scheduling document can be ... PDF Pharmacist's Manual Controlled Substances Act. Revised 2022. 1. ... Control Division, as a guide to assist pharmacists in their understanding of the Federal Controlled ... This Pharmacist's Manual is intended to summarize and explain the basic requirements for prescribing, administering, and dispensing controlled substances under the Controlled Substances ... Drug Scheduling & Classifications (List of Schedule I-V Controlled Drugs) Heroin, LSD, Marijuana, Ecstasy, Quaaludes, Bath salts, Suboxone, Ketamine, Anabolic steroids, Robitussin AC, Ezogabine, Its actual or relative potential for abuse. Scientific evidence of its pharmacological effect, if known. The state of current scientific knowledge regarding the drug or other substance. Its history and current pattern of abuse.
Explain how controlled substances are identified on their labels. Prescription of Controlled Substances: Benefits and Risks The Controlled Substance Act covers drug: Classification and regulation, according to their content and purpose. Manufacturing, Distribution, Exportation and sale, The Controlled Substance Act established five drug schedules and classified them to control their manufacture and distribution. Controlled Substance Schedules - United States Department of Justice Drugs and other substances that are considered controlled substances under the Controlled Substances Act (CSA) are divided into five schedules. An updated and complete list of the schedules is published annually in Title 21 Code of Federal Regulations (C.F.R.) §§1308.11 through 1308.15. Substances are placed in their respective schedules ... Code of Laws - Title 44 - Chapter 53 - Poisons, Drugs, And ... SECTION 44-53-10. General powers of Department of Health and Environmental Control regarding controlled substances. The Department of Health and Environmental Control shall take cognizance of the interest of the public health as it relates to the sale of drugs and the adulteration thereof and shall make all necessary inquiries and investigations relating thereto. Implementation Guidelines for Alcohol and Drug Regulations ... The FMCSA regulation requires testing for the following controlled substances (or their metabolites): marijuana, cocaine, opiates, phencyclidine (PCP), and amphetamines. The regulatory requirement is found in 49 CFR, section 40.85. You may test for additional controlled substances under your own authority provided that. 1.
WHMIS 1988 - Labelling Requirements : OSH Answers - Canadian Centre for ... appear on all controlled products produced in a workplace or transferred to other containers by the employer, may appear in placard form on controlled products received in bulk from a supplier, have the following information: product identifier (product name) information for the safe handling of the product, statement that the MSDS is available, Chapter 51- Principles of Pharmacology Flashcards | Quizlet controlled substance, a drug that is categorized as potentially dangerous or addictive, magnetic therapy, the use of magnets of various shapes, dispense, distribute a drug to a patient who is to use it, pharmacology, the science or study of drugs, labeling, A statement of the forms of a drug and its approved indications, e-prescribing, eCFR :: 16 CFR Part 1500 -- Hazardous Substances and Articles ... Because these guidelines apply to hazardous substances in general as well as to hazardous substances in art materials, the guidelines are set forth in § 1500.135 and a definition of “chronic toxicity” is provided in § 1500.3(c)(2)(ii) as part of supplementation of the term “toxic” in section 2(q) of the FHSA. Controlled Substances Program | FDA The Controlled Substances Program (CSP) is overseen by the Associate Director for Controlled Substances and aims to promote the public health by minimizing risks associated with problematic use of ...
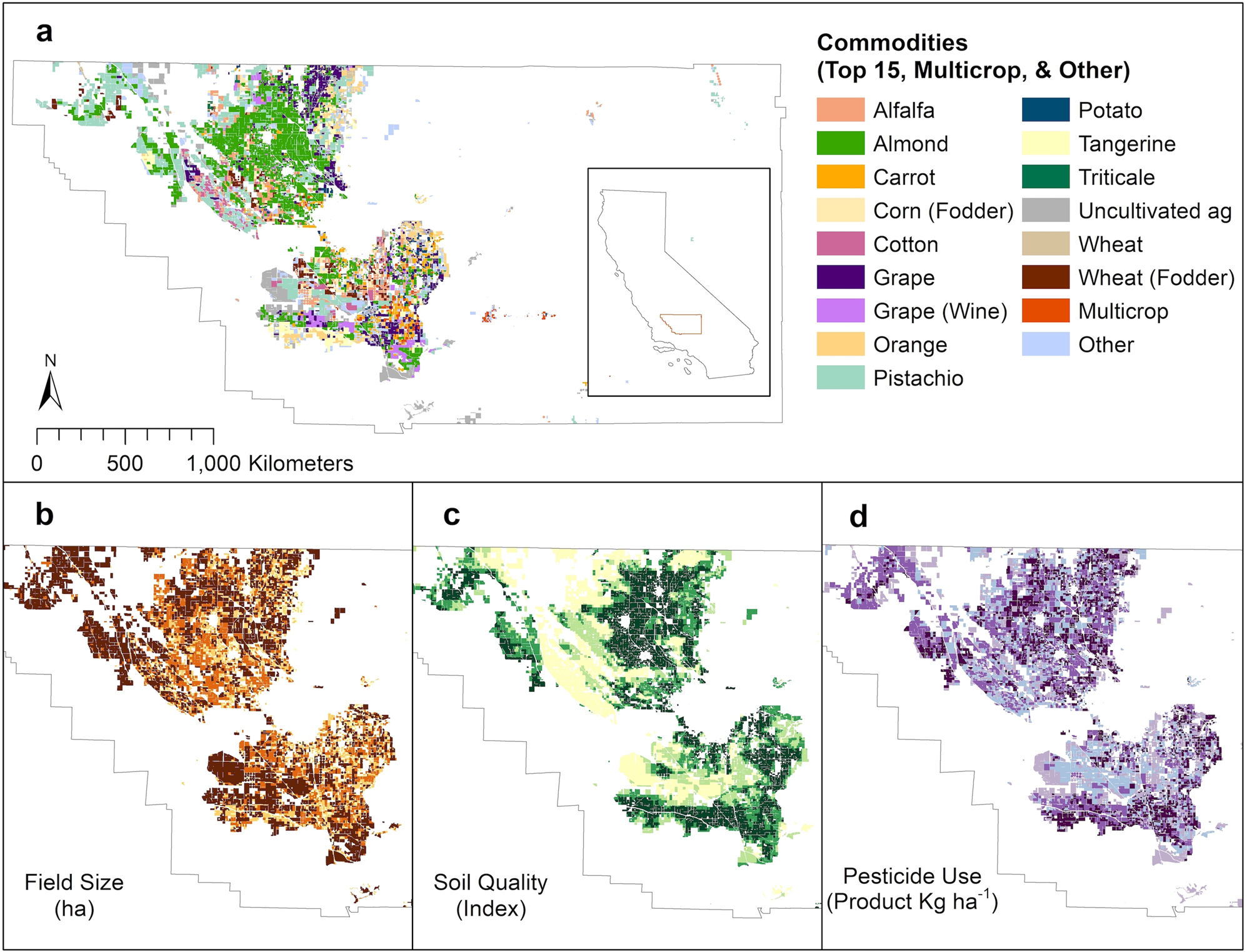
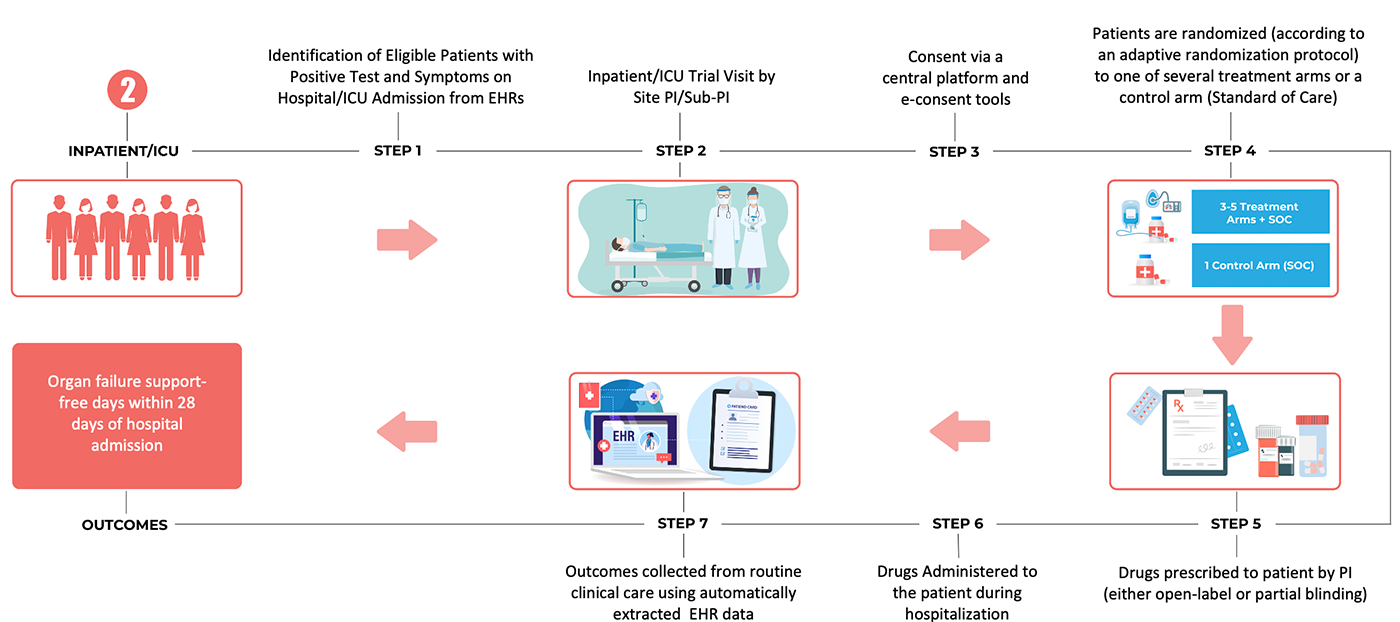
How to Read Over-the-Counter and Prescription Drug Labels - Drugwatch.com Check the label to make sure your name is on it. If it isn't, talk to the pharmacist. Check the label to make sure you can read and understand the name of the medicine, directions and colored warning stickers on the package. If the letters are too small to read, ask your pharmacist to print it in a larger type. Anabolic steroid - Wikipedia The history of the U.S. legislation on AAS goes back to the late 1980s, when the U.S. Congress considered placing AAS under the Controlled Substances Act following the controversy over Ben Johnson's victory at the 1988 Summer Olympics in Seoul. AAS were added to Schedule III of the Controlled Substances Act in the Anabolic Steroids Control Act ... GHS Hazard Classification: Everything You Need to Know - ERA Environmental T his article is part of ERA's three part series on GHS Hazard Classification.Part one outlines the step-by-step process for classifying your hazardous chemicals. Under the new Globally Harmonized System (GHS) of SDS and Label authoring, chemical manufacturers, importers, and distributors are required to update the way they classify and communicate the hazards of their products. 4 Controlled Substance Laws and Regulations You Should Know Controlled substance prescriptions have specific requirements. All prescriptions for controlled substances must include the following: 2, Date prescription was issued, Prescriber's signature, Patient's full name and address, Medication name, Strength, Dosage form, Quantity prescribed, Directions for use,
Scheduling basics | Therapeutic Goods Administration (TGA) Scheduling is a national classification system that controls how medicines and chemicals are made available to the public. Medicines and chemicals are classified into Schedules according to the level of regulatory control over the availability of the medicine or chemical required to protect public health and safety. The Schedules are,
453 Controlled Substances and Drugs | Postal Explorer - USPS 453 Controlled Substances and Drugs 453.1 Definitions 453.11 Controlled Substances. A controlled substance is any anabolic steroid, narcotic, hallucinogenic, stimulant, or depressant drug identified in Schedules I through V of the Controlled Substances Act in 21 U.S.C. 801 and the implementing regulations in 21 CFR 1300.
National Drug Codes Explained: What You Need to Know - Drugs.com The NDC, or National Drug Code, is a unique 10-digit or 11-digit, 3-segment number, and a universal product identifier for human drugs in the United States. The 3 segments of the NDC identify: the labeler, the product, and the commercial package size.
What are Auxiliary Labels? - PTCB Test Prep Examples of common auxiliary labels include: Do not chew or crush, Swallow whole, Take with food or milk, For rectal use only, Shake well before use, For external use only, May cause drowsiness, Protect from sunlight, Take on an empty stomach, Keep refrigerated, For the eye (or ear) only, May cause urine discoloration,
28 Pa. Code Chapter 25. Controlled Substances, Drugs, Devices ... A. CONTROLLED SUBSTANCES, DRUGS, DEVICES AND COSMETICS … 25.1 B. HEARING AID SALES AND REGISTRATION … 25.201 Authority. The provisions of this Chapter 25 issued under the Controlled Substance, Drug, Device and Cosmetic Act (35 P. S. § § 780-101—780-144), unless otherwise noted.
Prescribing Controlled Substances | Baptist Health CME The Prescribing Controlled Substances course has been approved by the Florida Board of Medicine, Florida Board of Osteopathic Medicine, Florida Board of Dentistry and Florida Board of Podiatry as continuing medical education that meets the controlled substance bill (House Bill 21) requirements for biennial license renewal.
The Controlled Substances Act - DEA These factors are listed in Section 201 (c), [21 U.S.C. § 811 (c)] of the CSA as follows: (1) Its actual or relative potential for abuse. (2) Scientific evidence of its pharmacological effect, if known. (3) The state of current scientific knowledge regarding the drug or other substance. (4) Its history and current pattern of abuse.
Pharmaceutical Labeling 101: FDA Regulations Guide The substance is a biological product such as tissues, vaccines, or recombinant proteins. This list makes it quite clear that not all drugs are products that are sold in pharmacies. Toiletries or hand sanitizers are also products that the FDA will regulate. All these products require FDA-approved labeling.
Drug Classifications, Schedule I, II, III, IV, V - MedShadow Controlled drugs that are considered to have virtually no risk for addiction, abuse or harm are not scheduled. Examples of those would be insulin, blood pressure and cholesterol medicines. Often searched for are: Ketamine (schedule 3), tramadol (schedule 4), weed/marijuana/pot (schedule 5 though uncontrolled/legal in some states).
What Is a Controlled Substance? (DEA Drug Classifications) Controlled substances are drugs that are subject to strict government control because they may cause addiction or be misused. The government's control impacted how these substances are made, used, stored, and transported. Examples of controlled substances include: stimulants, opioids, hallucinogens, anabolic steroids, depressants,
Prescription Labels and Drug Safety - Consumer Reports Although the Federal Food, Drug, and Cosmetic Act requires certain details to appear on bottle labels (like the patient's name and dosage instructions), other details can vary by state. The labels...
Identification, Classification and Labelling of Chemicals ... All chemicals, both substances and preparations, should have a clear marking to indicate their identity. The packages and containers of dangerous substances and preparations should, in addition to marking only, to have a label with required information.
How to Label Prescription Medications | Study.com The information on the label includes: Name of pharmacy with address and phone number (ex. X Pharmacy; 5000 Texas Rd., Beach, CA 32760; 555-555-1234) Name and strength of drug including generic ...
Controlled substances Flashcards | Quizlet Define controlled substances, CS are drugs and other substances that have been determined by federal and state to have potential for creating an addiction or dependency and for leading to various forms of abuse. Many CS are not recognized as drugs. Controlled drugs can be prescription or nonprescription, How do federal and state control CS,
20 Questions and Answers | Ozone Secretariat Ozone-depleting substances (ODSs) are the subset of these gases emitted by human activities that are controlled by the Montreal Protocol. These partitioned columns show the abundances of chlorine- and bromine-containing gases entering the stratosphere in 1993 and 1998, when their total amounts peaked, respectively, and in 2016.
CSA Schedules - Drugs.com The Controlled Substances Act (CSA) schedule information displayed applies to substances regulated under federal law. There may be variations in CSA schedules between individual states. Schedule I. The drug, substance, or chemical has a high potential for abuse. The drug, substance, or chemical has no currently accepted medical use in treatment ...
Drug Scheduling & Classifications (List of Schedule I-V Controlled Drugs) Heroin, LSD, Marijuana, Ecstasy, Quaaludes, Bath salts, Suboxone, Ketamine, Anabolic steroids, Robitussin AC, Ezogabine, Its actual or relative potential for abuse. Scientific evidence of its pharmacological effect, if known. The state of current scientific knowledge regarding the drug or other substance. Its history and current pattern of abuse.
PDF Pharmacist's Manual Controlled Substances Act. Revised 2022. 1. ... Control Division, as a guide to assist pharmacists in their understanding of the Federal Controlled ... This Pharmacist's Manual is intended to summarize and explain the basic requirements for prescribing, administering, and dispensing controlled substances under the Controlled Substances ...
Controlled Substances - FAQ - Environmental Health and Safety What are controlled substances? Controlled substances are drugs or chemicals that have the potential to be addictive or habit-forming. The DEA divides controlled substances into 5 categories called Schedules based upon substances' potential for abuse and addictiveness and its' usefulness in medicine. The Drug Scheduling document can be ...




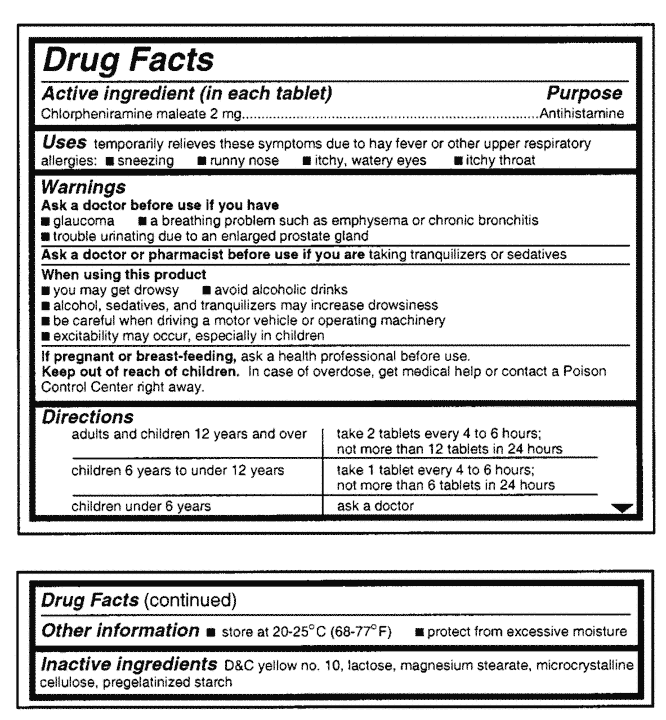

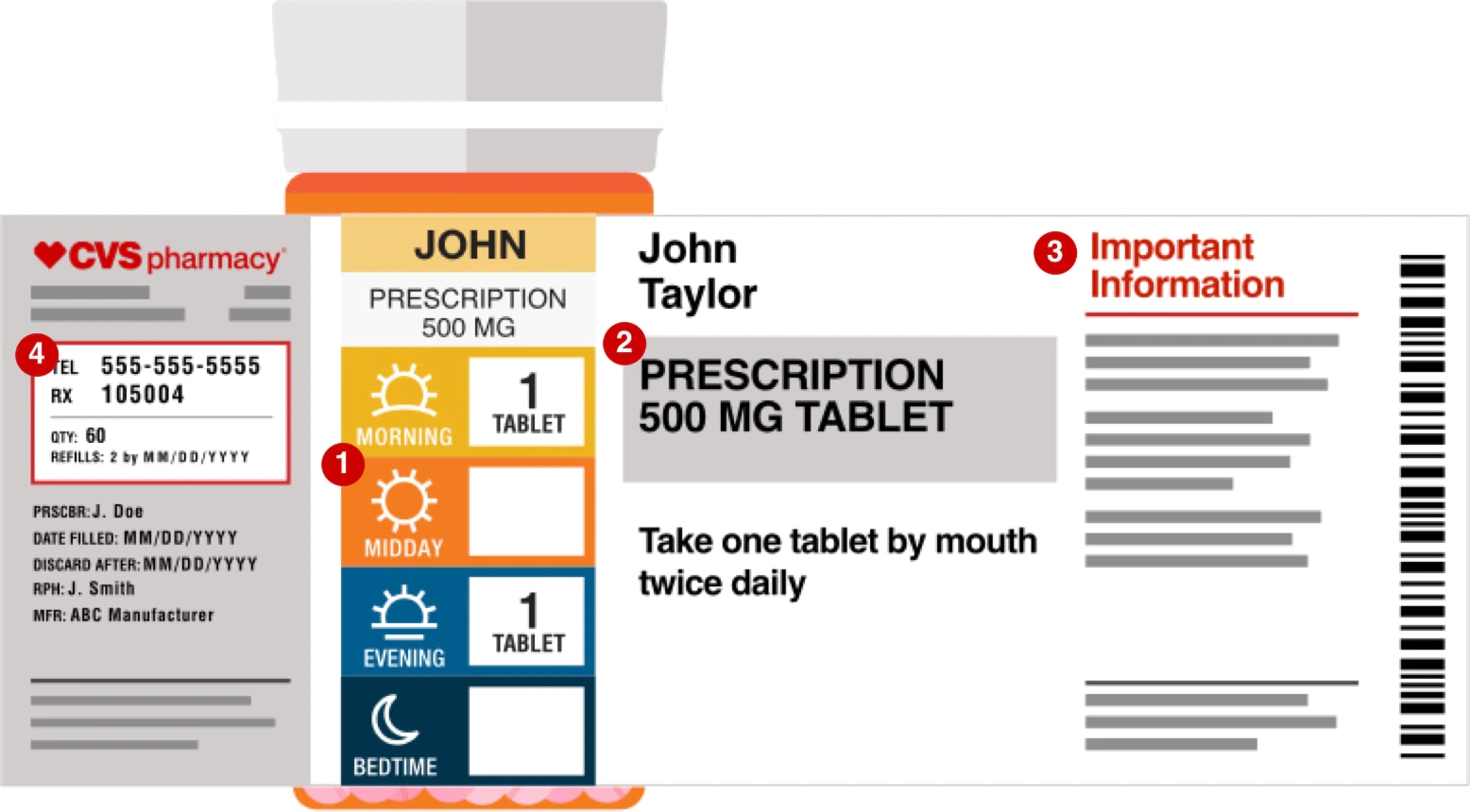
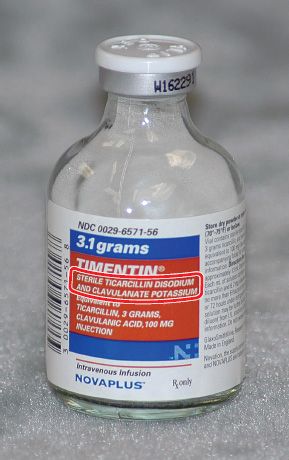





/3233264_color1-5c01876646e0fb00010cedb8.png)









Post a Comment for "38 explain how controlled substances are identified on their labels"